Zapping Away The Blues
A pacemaker like device to treat depression takes a giant step forward
This month Cyberonics Inc., in
Some 11 million such treatment-resistant patients
live in the developed world, more than 4 million of them in the
|
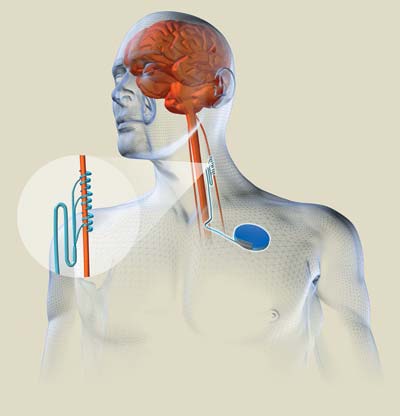
PSYCHIATRIC SYSTEM: A Vagus nerve stimulator implanted under the skin of the
chest transmits a train of electrical pulses along leads to the left Vagus
nerve in the neck. The nerve, in turn, conveys the signals to the brain,
thereby reducing the symptoms of depression.
|
About the size of a pocket watch, the nerve stimulator looks and acts much like a cardiac pacemaker, and it is implanted in the same place: under the skin of the chest. However, it sends electric pulses not to the heart but to the left vagus nerve in the. (Typically, it delivers 1- to 2-milliampere, 250-microsecond pulses at 20 to 30 hertz, for 30 seconds every 5 minutes.) The nerve regulates such diverse functions as heart rate and muscle tone in the gut. Two decades ago, scientists discovered that if they stimulated the nerve electrically, it prevented epileptic seizures. In 1997, Cyberonics' device was approved for that purpose. Now 30 000 epileptic patients around the world rely on it. It can be implanted in an outpatient setting.
Early on, some epilepsy patients reported that the device had also improved their mood, adding one more piece of evidence to the longstanding hypothesis of a neurological link between epilepsy and depression. A quarter of the people with epilepsy also have severe depression, according to a recent study. That rate far exceeds the prevalence of depression in people with other chronic conditions.
Phillip C. Jobe at the University of Illinois Medical Center at Chicago proposes that the brain's natural defenses against both seizures and depression are weakened by chemical and structural flaws in neurons that project out from brain structures called the dorsal raphe nucleus and the locus coeruleus and into other areas of the brain. Electrical stimulation of the vagus nerve alters activity in both those areas, although the nerve does not connect directly to either of them.
Six years ago, Cyberonics began depression trials in
the
After one year, one of six was free of depression, and 56 percent got some meaningful benefit. Of those who did respond, about 70 percent continued to benefit after two years
"I had no idea that life didn't have to have a dark veil over it all the time," she says. "And that you could actually look forward to next week or next month or next year." The only side effect she notices is a slight waver in her voice when the stimulator is on.
More than 400 people with depression participated in the trials. After one year, one of six was free of depression, and 56 percent got some meaningful benefit. Of those who did respond, about 70 percent continued to benefit after two years. But the FDA was initially skeptical of Cyberonics' results, and last year, in a rare move, it overrode its own advisory panel and rejected the device. But after high-level negotiations and the submission of supplemental data in the fall, the FDA reversed itself.
The agency's nod is, however, hedged with conditions
on a number of matters, including labeling, the maintenance of a patient
registry, quality of manufacturing, and protocols for a study to determine the
optimal dose—that is, the right amount of current. Robert P. ("Skip")
Cummins, Cyberonics' CEO, told investors he expects to get final approval in
time to introduce the device at the American Psychiatric Association meeting in
The population of potential users of the device for depression is 10 times as big as the one it already serves for epilepsy, and Cummins predicts that Cyberonics will be the first US $1 billion neuromodulation company. He bases his billion-dollar figure on the assumption that Cyberonics will capture just a small fraction of the new market and that its sales will grow as fast as its epilepsy treatment did in the late 1990s. The company's epilepsy business brings in revenues of $110 million per year and is growing at about 6 percent annually. So far, though, the company has not turned a profit, in part because it has plowed so much money into the depression trials.
Cash from the depression business should help Cyberonics explore other uses for its vagus-nerve stimulator, such as treatment of Alzheimer's disease, anxiety, chronic headache, and bulimia. The company also plans to investigate therapies involving the electrostimulation of other nerves. Its patents for such therapies are good until 2011, and Cummins says he expects that they can be extended to 2015.
Besides "talk therapies" and drugs, the only
other treatment for depression that is approved in the
Other electrically mediated treatments for depression
are under investigation. Neuronetics Inc., in
Such treatments present a curious twist on getting a prescription refilled. After six years of service, the battery in Karmen McGuffee's implant is nearing the end of its life. "I will definitely get it replaced," she says.
—SAMUEL K.