Brain Implants to Restore VisionA bionic implant that bypasses the eye might eventually treat many forms of blindness. By Duncan Graham-Rowe
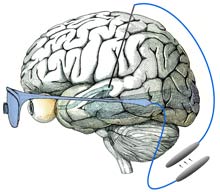
One day it may be possible to restore sight in people who are congenitally blind by placing an implant in a part of the vision system hitherto ignored. Unlike most visual prostheses in advanced development, this new approach could make it possible to treat blindness even when the entire eye is damaged. While the work is still in the early stages, researchers ultimately envision a device that translates images from a digital camera into neural impulses and then feeds that information into the visual system, allowing the wearer to see. Previous research has shown that visual sensations, known as percepts, can be elicited in blind subjects by electrically stimulating nerve cells within the vision system. Researchers at Harvard Medical School, in Boston, are designing a visual prosthesis that builds on that observation. Several types of sight-boosting prostheses are currently under development, with some already being tested in humans. But while these largely target the retina, the Harvard researchers chose to focus on part of the visual system called the lateral geniculate nucleus (LGN), a relay station along the route from the optic nerve to the visual cortex, where visual information is processed. Because it's upstream of the eye, this area could be targeted in people with extensive eye damage. And unlike locations in the visual cortex, the LGN is one of the first "stops" in the visual system, meaning that the neural signals encoding visual information have not yet been extensively processed and spread throughout the brain. "[In the LGN] there is a straightforward mapping of the visual scene on the tissue," says John Pezaris, a neural-systems engineer at Harvard Medical School, who co-authored the research along with neuroscientist Clay Reid, also at Harvard Medical School. This means that specific parts of the LGN are linked to specific parts of the visual scene. When a light flashes in one location, for example, the corresponding area in the LGN will become active. To determine if activity in the LGN can mimic visual stimuli, the researchers implanted electrodes in the LGNs of two monkeys that had been trained to move their eyes rapidly toward points of light when they appeared on a screen. When a part of the LGN corresponding to a specific part of the visual field was electrically stimulated, the monkeys would shift their gaze to that point on the screen. The results, published today in the Proceedings of the National Academy of Sciences, suggest that the monkeys were "seeing" the pulses in their field of view even though nothing was appearing on the screen. "It's an absolutely stunning piece of work," says James Morrison, a physiologist and principal investigator for the Retinal Prosthesis Group at the University of Glasgow's Institute of Biomedical and Life Sciences, in Scotland. However, Morrison says, the position of the LGN is a major disadvantage of the approach. It's located in the middle of the head, making it difficult to access. Recent advances in neurosurgical techniques, such as deep brain stimulators for treating Parkinson's disease, may help solve this issue: the LGN is just a few centimeters away from where these stimulators are placed, says Pezaris. Still, it's too soon to say whether the findings will lead to better brain implants. "While I think the paper holds scientific merit, I think it will be extremely difficult to restore blindness from there," says Thomas Serre, a neuroscientist at the Center for Biological and Computational Learning at MIT's McGovern Institute for Brain Research. He believes that neurons in the LGN may be spaced too closely together to be stimulated individually, which would be important in trying to reproduce natural vision. "I don't think we will ever be able to go beyond generating very simple percepts like points of light," he says. Pezaris accepts that a tremendous amount of work is needed before the LGN can be used to treat blindness, but he says this work does at least open the door to that possibility. "This was just the first very small step," he says.
|